
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
- Pathophysiology
- Rho-Kinase as a Therapeutic Target for Nonalcoholic Fatty Liver Diseases
- Inês Sousa-Lima, Hyun Jeong Kim, John Jones, Young-Bum Kim
- Diabetes Metab J. 2021;45(5):655-674. Published online September 30, 2021
- DOI: https://doi.org/10.4093/dmj.2021.0197
- 5,793 View
- 171 Download
- 7 Web of Science
- 7 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub 
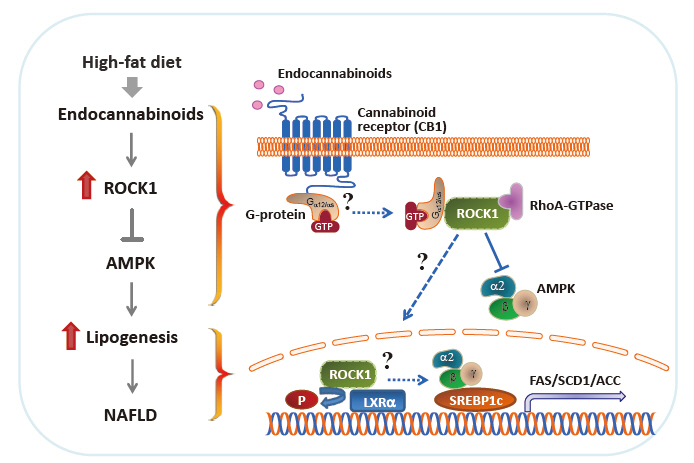
- Nonalcoholic fatty liver disease (NAFLD) is a major public health problem and the most common form of chronic liver disease, affecting 25% of the global population. Although NAFLD is closely linked with obesity, insulin resistance, and type 2 diabetes mellitus, knowledge on its pathogenesis remains incomplete. Emerging data have underscored the importance of Rho-kinase (Rho-associated coiled-coil-containing kinase [ROCK]) action in the maintenance of normal hepatic lipid homeostasis. In particular, pharmacological blockade of ROCK in hepatocytes or hepatic stellate cells prevents the progression of liver diseases such as NAFLD and fibrosis. Moreover, mice lacking hepatic ROCK1 are protected against obesity-induced fatty liver diseases by suppressing hepatic de novo lipogenesis. Here we review the roles of ROCK as an indispensable regulator of obesity-induced fatty liver disease and highlight the key cellular pathway governing hepatic lipid accumulation, with focus on de novo lipogenesis and its impact on therapeutic potential. Consequently, a comprehensive understanding of the metabolic milieu linking to liver dysfunction triggered by ROCK activation may help identify new targets for treating fatty liver diseases such as NAFLD.
-
Citations
Citations to this article as recorded by- THE ROLE OF N6-METHYLADENOSINE METHYLTRANSFERASE RBM15 IN NONALCOHOLIC FATTY LIVER DISEASE
Shiqing Li, Shengyi Lian, Wei Cheng, Tao Zhang, Xiaobing Gong
Shock.2024; 61(2): 311. CrossRef - Exploring the potential of drug repurposing for liver diseases: A comprehensive study
Fares E.M. Ali, Mustafa Ahmed Abdel-Reheim, Emad H.M. Hassanein, Mostafa K. Abd El-Aziz, Hanan S. Althagafy, Khalid S.A. Badran
Life Sciences.2024; : 122642. CrossRef - Targeting of G-protein coupled receptor 40 alleviates airway hyperresponsiveness through RhoA/ROCK1 signaling pathway in obese asthmatic mice
Xixi Lin, Like Wang, Xiaojie Lu, Yuanyuan Zhang, Rongying Zheng, Ruijie Chen, Weixi Zhang
Respiratory Research.2023;[Epub] CrossRef - Selectivity matters: selective ROCK2 inhibitor ameliorates established liver fibrosis via targeting inflammation, fibrosis, and metabolism
Alexandra Zanin-Zhorov, Wei Chen, Julien Moretti, Melanie S. Nyuydzefe, Iris Zhorov, Rashmi Munshi, Malavika Ghosh, Cindy Serdjebi, Kelli MacDonald, Bruce R. Blazar, Melissa Palmer, Samuel D. Waksal
Communications Biology.2023;[Epub] CrossRef - Insight Into Rho Kinase Isoforms in Obesity and Energy Homeostasis
Lei Wei, Jianjian Shi
Frontiers in Endocrinology.2022;[Epub] CrossRef - Paeoniflorin alleviates liver injury in hypercholesterolemic rats through the ROCK/AMPK pathway
Tong Liu, Ning Zhang, Lingya Kong, Sijie Chu, Ting Zhang, Guangdi Yan, Donglai Ma, Jun Dai, Zhihong Ma
Frontiers in Pharmacology.2022;[Epub] CrossRef - Fasudil Increased the Sensitivity to Gefitinib in NSCLC by Decreasing Intracellular Lipid Accumulation
Tingting Liao, Jingjing Deng, Wenjuan Chen, Juanjuan Xu, Guanghai Yang, Mei Zhou, Zhilei Lv, Sufei Wang, Siwei Song, Xueyun Tan, Zhengrong Yin, Yumei Li, Yang Jin
Cancers.2022; 14(19): 4709. CrossRef
- THE ROLE OF N6-METHYLADENOSINE METHYLTRANSFERASE RBM15 IN NONALCOHOLIC FATTY LIVER DISEASE
- Lifesytle
- Combined Aerobic and Resistance Exercise Training Reduces Circulating Apolipoprotein J Levels and Improves Insulin Resistance in Postmenopausal Diabetic Women
- Yun Kyung Jeon, Sang Soo Kim, Jong Ho Kim, Hyun Jeong Kim, Hyun Jun Kim, Jang Jun Park, Yuen Suk Cho, So Hee Joung, Ji Ryang Kim, Bo Hyun Kim, Sang Heon Song, In Joo Kim, Yong Ki Kim, Young-Bum Kim
- Diabetes Metab J. 2020;44(1):103-112. Published online February 21, 2020
- DOI: https://doi.org/10.4093/dmj.2018.0160
- 8,488 View
- 153 Download
- 12 Web of Science
- 12 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader Background Circulating apolipoprotein J (ApoJ) is closely associated with insulin resistance; however, the effect of exercise on circulating ApoJ levels and the association of ApoJ with metabolic indices remain unknown. Here, we investigated whether a combined exercise can alter the circulating ApoJ level, and whether these changes are associated with metabolic indices in patients with type 2 diabetes mellitus.
Methods Postmenopausal women with type 2 diabetes mellitus were randomly assigned into either an exercise (EXE,
n =30) or control (CON,n =15) group. Participants in the EXE group were enrolled in a 12-week program consisting of a combination of aerobic and resistance exercises. At baseline, 4, 8, and 12 weeks, body composition and metabolic parameters including homeostatic model assessment of insulin resistance (HOMA-IR) and serum ApoJ levels were assessed.Results In the EXE group, ApoJ levels decreased 26.3% and 19.4%, relative to baseline, at 8 and 12 weeks, respectively. Between-group differences were significant at 8 and 12 weeks (
P <0.05 andP <0.001, respectively). In the EXE group, 12 weeks of exercise resulted in significant decreases in body weight, percent body fat, and HOMA-IR indices. Concurrently, weight-adjusted appendicular skeletal muscle mass (ASM/wt) was increased in the EXE group compared with the CON group. Importantly, changes in the ApoJ level were significantly correlated with changes in ASM/wt.Conclusion Exercise training resulted in a significant decrease in the circulating ApoJ level, with changes in ApoJ associated with an improvement in some insulin resistance indices. These data suggest that circulating ApoJ may be a useful metabolic marker for assessing the effects of exercise on insulin resistance.
-
Citations
Citations to this article as recorded by- The function of previously unappreciated exerkines secreted by muscle in regulation of neurodegenerative diseases
Xuepeng Bian, Qian Wang, Yibing Wang, Shujie Lou
Frontiers in Molecular Neuroscience.2024;[Epub] CrossRef - Exercise modalities for type 2 diabetes: A systematic review and network meta‐analysis of randomized trials
Liangying Hou, Qi Wang, Bei Pan, Rui Li, Yanfei Li, Juanjuan He, Tianzhu Qin, Liujiao Cao, Na Zhang, Changhao Cao, Long Ge, Kehu Yang
Diabetes/Metabolism Research and Reviews.2023;[Epub] CrossRef - Estimating the Effect of Aerobic Exercise Training on Novel Lipid Biomarkers: A Systematic Review and Multivariate Meta-Analysis of Randomized Controlled Trials
Gina Wood, Emily Taylor, Vanessa Ng, Anna Murrell, Aditya Patil, Tom van der Touw, Mitch Wolden, Nick Andronicos, Neil A. Smart
Sports Medicine.2023; 53(4): 871. CrossRef - 2023 update on Italian guidelines for the treatment of type 2 diabetes
Edoardo Mannucci, Riccardo Candido, Lina delle Monache, Marco Gallo, Andrea Giaccari, Maria Luisa Masini, Angela Mazzone, Gerardo Medea, Basilio Pintaudi, Giovanni Targher, Marina Trento, Giuseppe Turchetti, Valentina Lorenzoni, Matteo Monami
Acta Diabetologica.2023; 60(8): 1119. CrossRef - The Effect of Eight Weeks of Concurrent Training on Serum Levels of Paraxonase-1, Irisin, Lipid Profile, and Insulin Resistance in Men With Metabolic Syndrome
Seyed Amir Hosain Diba Hosaini, Morvarid Vafaee, Bahram Abedi
Hormozgan Medical Journal.2023; 27(1): 43. CrossRef - An Overview of the TRP-Oxidative Stress Axis in Metabolic Syndrome: Insights for Novel Therapeutic Approaches
Mizael C. Araújo, Suzany H. S. Soczek, Jaqueline P. Pontes, Leonardo A. C. Marques, Gabriela S. Santos, Gisele Simão, Laryssa R. Bueno, Daniele Maria-Ferreira, Marcelo N. Muscará, Elizabeth S. Fernandes
Cells.2022; 11(8): 1292. CrossRef - Effect of Yijinjing combined with elastic band exercise on muscle mass and function in middle-aged and elderly patients with prediabetes: A randomized controlled trial
Yunda Huang, Junhua Han, Qing Gu, Yanwei Cai, Jingyuan Li, Shasha Wang, Suijun Wang, Ru Wang, Xiangyun Liu
Frontiers in Medicine.2022;[Epub] CrossRef - Effect of combined aerobic and resistance exercise on blood pressure in postmenopausal women: A systematic review and meta-analysis of randomized controlled trials
Huihui Xi, Yayu He, Yirou Niu, Xin Sui, Jun Zhang, Ruiting Zhu, Haiyan Xu, Shuang Zhang, Yang Li, Yuan Yuan, Lirong Guo
Experimental Gerontology.2021; 155: 111560. CrossRef - Effects of Augmented-Reality-Based Exercise on Muscle Parameters, Physical Performance, and Exercise Self-Efficacy for Older Adults
Sangwan Jeon, Jiyoun Kim
International Journal of Environmental Research and Public Health.2020; 17(9): 3260. CrossRef - Apolipoprotein J is a hepatokine regulating muscle glucose metabolism and insulin sensitivity
Ji A Seo, Min-Cheol Kang, Won-Mo Yang, Won Min Hwang, Sang Soo Kim, Soo Hyun Hong, Jee-In Heo, Achana Vijyakumar, Leandro Pereira de Moura, Aykut Uner, Hu Huang, Seung Hwan Lee, Inês S. Lima, Kyong Soo Park, Min Seon Kim, Yossi Dagon, Thomas E. Willnow, V
Nature Communications.2020;[Epub] CrossRef - Impact of Skeletal Muscle Mass on Metabolic Health
Gyuri Kim, Jae Hyeon Kim
Endocrinology and Metabolism.2020; 35(1): 1. CrossRef - Habitual Combined Exercise Protects against Age-Associated Decline in Vascular Function and Lipid Profiles in Elderly Postmenopausal Women
Elizabeth J. Pekas, John Shin, Won-Mok Son, Ronald J. Headid, Song-Young Park
International Journal of Environmental Research and Public Health.2020; 17(11): 3893. CrossRef
- The function of previously unappreciated exerkines secreted by muscle in regulation of neurodegenerative diseases
- Pathophysiology
- Metformin Ameliorates Lipotoxic β-Cell Dysfunction through a Concentration-Dependent Dual Mechanism of Action
- Hong Il Kim, Ji Seon Lee, Byung Kook Kwak, Won Min Hwang, Min Joo Kim, Young-Bum Kim, Sung Soo Chung, Kyong Soo Park
- Diabetes Metab J. 2019;43(6):854-866. Published online June 27, 2019
- DOI: https://doi.org/10.4093/dmj.2018.0179
- 6,660 View
- 115 Download
- 14 Web of Science
- 13 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Chronic exposure to elevated levels of free fatty acids contributes to pancreatic β-cell dysfunction. Although it is well known that metformin induces cellular energy depletion and a concomitant activation of AMP-activated protein kinase (AMPK) through inhibition of the respiratory chain, previous studies have shown inconsistent results with regard to the action of metformin on pancreatic β-cells. We therefore examined the effects of metformin on pancreatic β-cells under lipotoxic stress.
Methods NIT-1 cells and mouse islets were exposed to palmitate and treated with 0.05 and 0.5 mM metformin. Cell viability, glucose-stimulated insulin secretion, cellular adenosine triphosphate, reactive oxygen species (ROS) levels and Rho kinase (ROCK) activities were measured. The phosphorylation of AMPK was evaluated by Western blot analysis and mRNA levels of endoplasmic reticulum (ER) stress markers and NADPH oxidase (NOX) were measured by real-time quantitative polymerase chain reaction analysis.
Results We found that metformin has protective effects on palmitate-induced β-cell dysfunction. Metformin at a concentration of 0.05 mM inhibits NOX and suppresses the palmitate-induced elevation of ER stress markers and ROS levels in a AMPK-independent manner, whereas 0.5 mM metformin inhibits ROCK activity and activates AMPK.
Conclusion This study suggests that the action of metformin on β-cell lipotoxicity was implemented by different molecular pathways depending on its concentration. Metformin at a usual therapeutic dose is supposed to alleviate lipotoxic β-cell dysfunction through inhibition of oxidative stress and ER stress.
-
Citations
Citations to this article as recorded by- Metformin enhances METTL14-Mediated m6A methylation to alleviate NIT-1 cells apoptosis induced by hydrogen peroxide
Si-min Zhou, Xin-ming Yao, Yi Cheng, Yu-jie Xing, Yue Sun, Qiang Hua, Shu-jun Wan, Xiang-jian Meng
Heliyon.2024; 10(2): e24432. CrossRef - Reduced Expression Level of Protein PhosphatasePPM1EServes to Maintain Insulin Secretion in Type 2 Diabetes
Sevda Gheibi, Luis Rodrigo Cataldo, Alexander Hamilton, Mi Huang, Sebastian Kalamajski, Malin Fex, Hindrik Mulder
Diabetes.2023; 72(4): 455. CrossRef - Metformin restores prohormone processing enzymes and normalizes aberrations in secretion of proinsulin and insulin in palmitate‐exposed human islets
Quan Wen, Azazul Islam Chowdhury, Banu Aydin, Mudhir Shekha, Rasmus Stenlid, Anders Forslund, Peter Bergsten
Diabetes, Obesity and Metabolism.2023; 25(12): 3757. CrossRef - Treatment of type 2 diabetes mellitus with stem cells and antidiabetic drugs: a dualistic and future-focused approach
Priyamvada Amol Arte, Kanchanlata Tungare, Mustansir Bhori, Renitta Jobby, Jyotirmoi Aich
Human Cell.2023; 37(1): 54. CrossRef - Metformin disrupts insulin secretion, causes proapoptotic and oxidative effects in rat pancreatic beta‐cells in vitro
Maíra M.R. Valle, Eloisa Aparecida Vilas‐Boas, Camila F. Lucena, Simone A. Teixeira, Marcelo N. Muscara, Angelo R. Carpinelli
Journal of Biochemical and Molecular Toxicology.2022;[Epub] CrossRef - Protection by metformin against severe Covid-19: An in-depth mechanistic analysis
Nicolas Wiernsperger, Abdallah Al-Salameh, Bertrand Cariou, Jean-Daniel Lalau
Diabetes & Metabolism.2022; 48(4): 101359. CrossRef - Insight Into Rho Kinase Isoforms in Obesity and Energy Homeostasis
Lei Wei, Jianjian Shi
Frontiers in Endocrinology.2022;[Epub] CrossRef - Overexpression of miR-297b-5p Promotes Metformin-Mediated Protection Against Stearic Acid-Induced Senescence by Targeting Igf1r
Qingrui Zhao, Shenghan Su, Yuqing Lin, Xuebei Li, Lingfeng Dan, Yunjin Zhang, Chunxiao Yang, Xiaohan Li, Yimeng Dong, Chenchen Geng, Changhao Sun, Xia Chu, Huimin Lu
SSRN Electronic Journal .2022;[Epub] CrossRef - Metformin Dysregulates the Unfolded Protein Response and the WNT/β-Catenin Pathway in Endometrial Cancer Cells through an AMPK-Independent Mechanism
Domenico Conza, Paola Mirra, Gaetano Calì, Luigi Insabato, Francesca Fiory, Francesco Beguinot, Luca Ulianich
Cells.2021; 10(5): 1067. CrossRef - NADPH Oxidase (NOX) Targeting in Diabetes: A Special Emphasis on Pancreatic β-Cell Dysfunction
Suma Elumalai, Udayakumar Karunakaran, Jun-Sung Moon, Kyu-Chang Won
Cells.2021; 10(7): 1573. CrossRef - Metformin use and cardiovascular outcomes in patients with diabetes and chronic kidney disease: a nationwide cohort study
Min Ho Kim, Hyung Jung Oh, Soon Hyo Kwon, Jin Seok Jeon, Hyunjin Noh, Dong Cheol Han, Hyoungnae Kim, Dong-Ryeol Ryu
Kidney Research and Clinical Practice.2021; 40(4): 660. CrossRef - Different Effects of Metformin and A769662 on Sodium Iodate-Induced Cytotoxicity in Retinal Pigment Epithelial Cells: Distinct Actions on Mitochondrial Fission and Respiration
Chi-Ming Chan, Ponarulselvam Sekar, Duen-Yi Huang, Shu-Hao Hsu, Wan-Wan Lin
Antioxidants.2020; 9(11): 1057. CrossRef - Metformin Reduces Lipotoxicity-Induced Meta-Inflammation in β-Cells through the Activation of GPR40-PLC-IP3 Pathway
Ximei Shen, Beibei Fan, Xin Hu, Liufen Luo, Yuanli Yan, Liyong Yang
Journal of Diabetes Research.2019; 2019: 1. CrossRef
- Metformin enhances METTL14-Mediated m6A methylation to alleviate NIT-1 cells apoptosis induced by hydrogen peroxide

 KDA
KDA
 First
First Prev
Prev





